Explain Four Different Types of Chemical Reaction With Suitable Examples
This is an example of a combustion reaction a redox process. Types of Chemical Reactions With Examples When you mix chemicals you may get a chemical reaction.

Types Of Chemical Reactions Youtube
Manganese IV oxide.

. Maximum time- 45 minutes. How is exothermic reaction different from an endothermic reaction. The following are the three main types of breakdown reactions.
In this equation Fe and H as well as O and Cl trade places. Atoms with relatively similar electronegativities share electrons between them and are connected by covalent bonds. Some chemical reactions are characterised by change in colour.
As a side note both solvents and catalysts may act as reagents but not as reactants since the former are not consumed during a chemical reaction. If youre asked the five main types of reactions it is these four and then either acid-base or redox depending who you ask. It is a type of continuous ecosystem Reaction in a step-by-step process to create multiple stable and liquid molecules later called abstract.
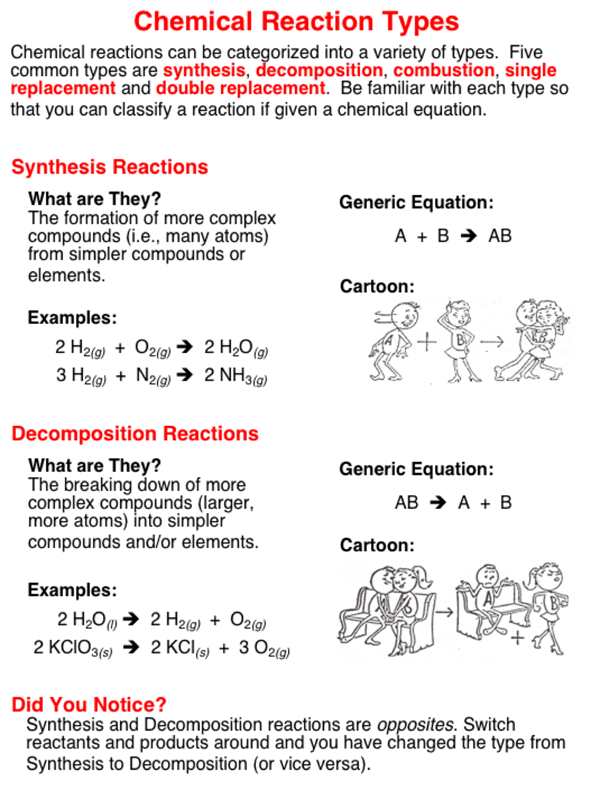
Types of Chemical Reactions. Oxidation numbers are arbitrary numbers based upon the following rules. Examples of other classes of reactions include oxidation-reduction redox reactions acid-base reactions complexation reactions and precipitation reactions.
How do we balance a chemical equation. Chemical bonds include covalent polar covalent and ionic bonds. Another example of a double displacement reaction is the reaction of leadII nitrate with potassium iodide to form leadII iodide and potassium nitrate.
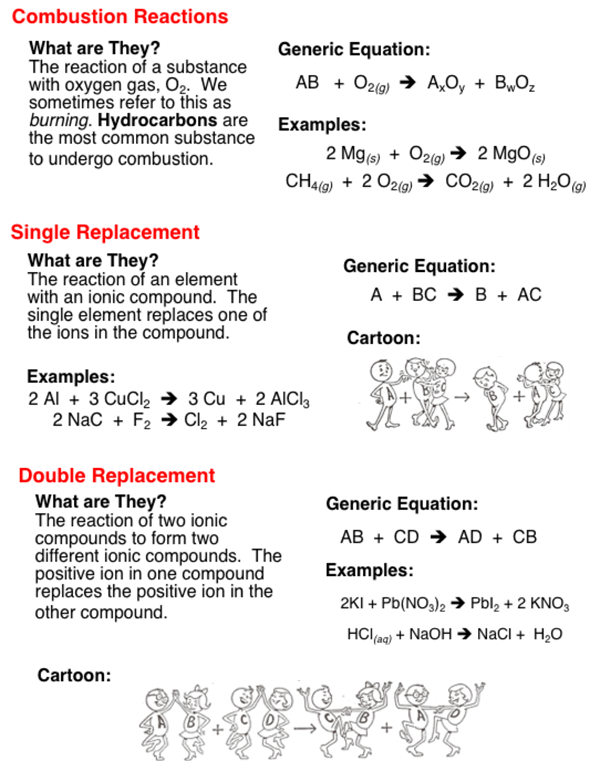
For example when barium chloride BaCl 2 and magnesium sulfate MgSO 4 react the SO 4 2 anion switches places with the 2Cl anion giving the compounds BaSO 4 and MgCl 2. What are the 4 types of organic reactions. Substitutions are the characteristic reactions of saturated compounds such as alkanes and alkyl halides and of aromatic compounds even though they are unsaturated.
Combustion reactions always involve oxygen and an organic fuel. The basis for different types of reactions is the product formed the changes that occur the reactants involved and so on. Finally inorganic chemical changes are chemical changes that dont use carbon as a part of the chemical reaction.
Reagents are added to a solution in order to test for or cause a reaction. Three examples of endothermic reactions are. The reaction in which two or more than two substance combine together to form a single compound.
Combustion reaction of methane. 2When sulphur dioxide gas reacts with acidified potassium dichromatechange in colour from orange to yellow is observed. 2 M g O 2 2 M g O.
Explain four different types of chemical reaction with suitable examples. Fe 2 O 3 6 HCl 2 FeCl 3 3 H 2 O. How is exothermic reaction different from an endothermic reaction.
Examples of organic chemical changes include halogenation methylation crude oil cracking and polymerization. Substances are either chemical elements or compounds. Four types of organic reactions are.
The oxidation number of uncombined elements is zero. Melting of ice 2. Sublimation of ammonium chloride 3.
Explain the process of corrosion and rusting. 7 rows Different Types of Chemical Reactions. The main four types of reactions are direct combination analysis reaction single displacement and double displacement.
In the following image we see methane combusting to release energy. CuSO 4 aqZnsZnSO 4 aqCus In this reaction zinc displaces copper from copper sulphate solution. Substitutions additions eliminations or rearrangements.
Precipitation or Double-Displacement Reaction. Chemical reactions are an integral part of technology of. Explain the process of corrosion and rusting.
Inorganic changes are processes like redox reactions oxidation and the mixture of bases and acids. Types of Chemical Reaction. Three important rearrangement Reactions are 12-rearrangements olefin metathesis and pericyclic Reactions.
Because there are so many reactions there are additional ways to categorize them but these other classes will still fall into one of the four main groups. Synthesis reactions happen when two separate atoms or molecules come together to generate a new molecule or substance. Atoms with large differences in electronegativity transfer electrons to form ions.
For Ex1When citric acid reacts with potassium permanganate purple then purple colour disappears. Different types of chemical reaction are. The 5 primary types of chemical reactions are.
How do we balance a chemical equation. What are three examples of endothermic reactions. Virtually all organic reactions fall into one of four categories.
A double displacement reaction usually occurs in solution and one of the products being. A chemical reaction rearranges the constituent atoms of the reactants to create different substances as products. Chemical reaction a process in which one or more substances the reactants are converted to one or more different substances the products.
Keep in mind a specific chemical reaction may fall into more than one category. Learn about the different types of chemical reactions and get examples of the reaction types. Those reactions in which two compounds by the exchange of ions to form two new compound are called double displacement reactions.
Reaction of Thermal Decomposition. Chemical bonds are forces that hold atoms together to make compounds or molecules. Explain four different types of chemical reaction with suitable examples.
Different types of reactions are. 2 H_2g O_2g rightarrow 2 H_2Og Decomposition Reactions.

Types Of Chemical Reactions Detailed Explanation With Example Videos
Comments
Post a Comment